TOP
Quality Inspection Study Team
Quality Inspection Study Team
This is our Quality Inspection Research Center for Global Cosmetics to ensure quality and safety of the world-class biotech industry.
Team Overview
Our Quality Inspection Research Center for Global Cosmetics aims to make contributions to securing quality and safety in cosmetic products distributed in and outside of Korea pursuant to the Cosmetics Act, its Enforcement Decree, and guidelines promulgated by the Ministry of Food and Drug Safety. Our research center has high-performance instruments in the wake of the development of science and technology and competent researchers who would lead the latest analytical techniques.
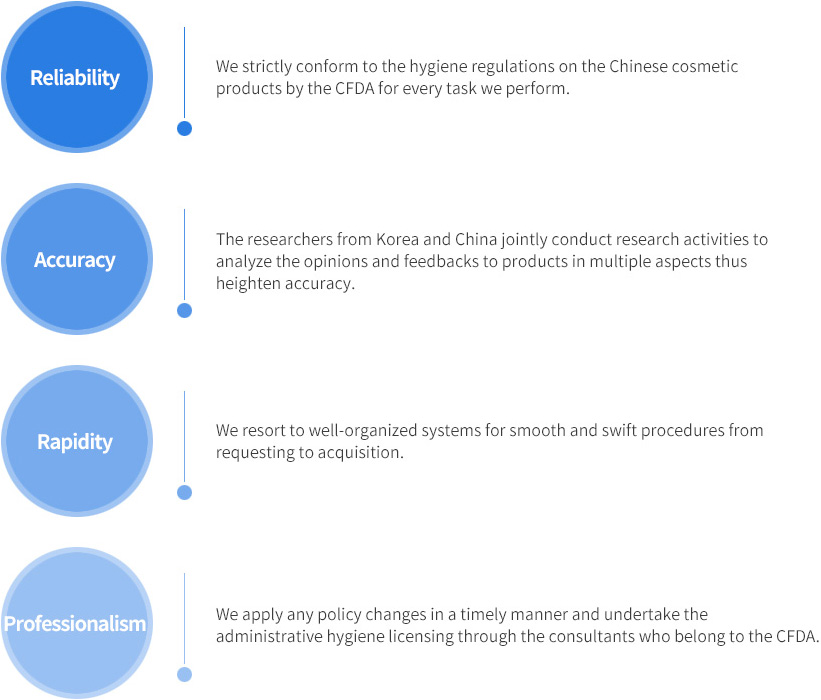
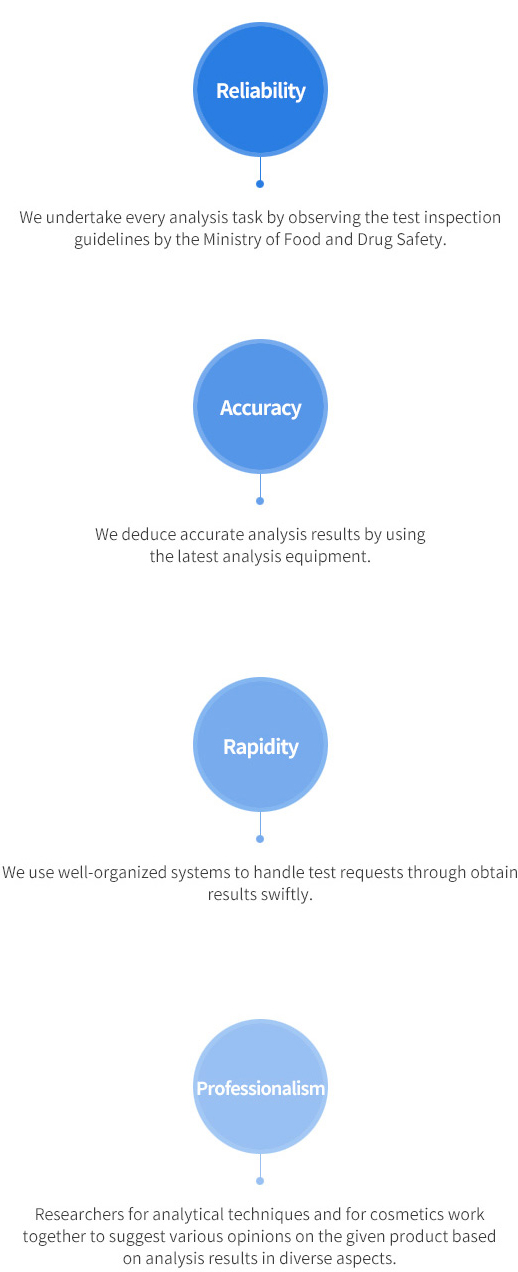
Pledge by Quality Inspection Research Center for Global Cosmetics
Cosmetics quality inspection service procedure
-
Submit analysis request
Online submission, submission by phone, field submission, etc.
-
Submit application
Test request, copy of business registration certificate and other test data
-
Counseling for test
Sample amount, test period, test fee, test method, test items
- Submit sample & pay fee
-
Establish test plan
Establish analysis schedule. Organize analysis team
-
Follow-up
measureNotify an action to take in case of analysis error or deviation. Set warranty period -
Notify
analysis reportBy post, visiting, fax, e-mail, etc. -
Draw up report at
the end of analysisYield results. Draw up report - Perform
analysis -
Meeting
before analysisRequired equipment, reagent, test solution and standard item preparation
-
Meeting before analysis
Required equipment, reagent, test solution and standard item preparation
- Perform analysis
-
Draw up report at the end of analysis
Yield results. Draw up report
-
Notify analysis report
By post, visiting, fax, e-mail, etc.
-
Follow-up measure
Notify an action to take in case of analysis error or deviation. Set warranty period
