TOP
Cosmetics Efficacy Clinical Study Team
Cosmetics Efficacy Clinical Study Team
Conduct evaluation on the functional cosmetics, quasi-drugs, antibacterial function, in addition to stability and effectiveness test on the cosmetic ingredients at once!
Evaluation services
Evaluation on antibacterial function, the functional cosmetics, dietary supplements, quasi-drugs, in addition to stability and effectiveness test on the cosmetic ingredients all at once!
Korea Institute of Dermatological Sciences undertakes the one-stop procedure on the non-clinical and clinical tests and analysis. Each evaluation service involves evaluations in light of usage and preference and provided results are eligible for submission to the Ministry of Food and Drug Safety
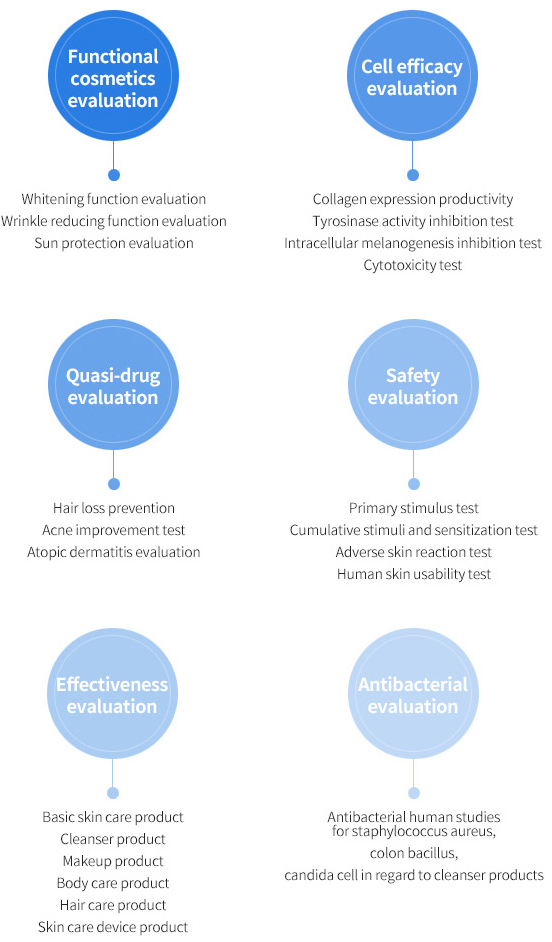
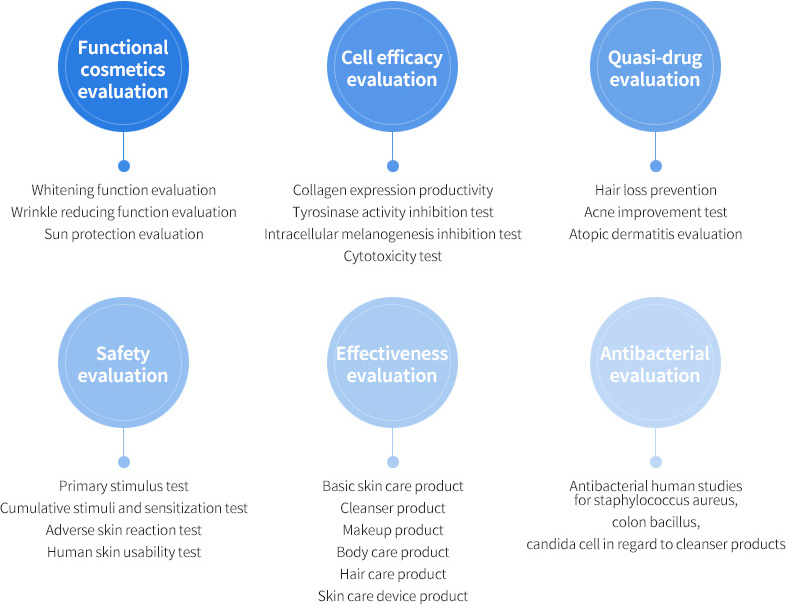
IRB (Institutional Review Board) is our independent clinical test review committee to ensure safety and rights of testees for human studies. Korea Institute of Dermatological Sciences(KIDS) appoint the experts in the fields of ethics, science and medical science as the IRB members to go through the strict review process for our research activities as a whole.
